Case study
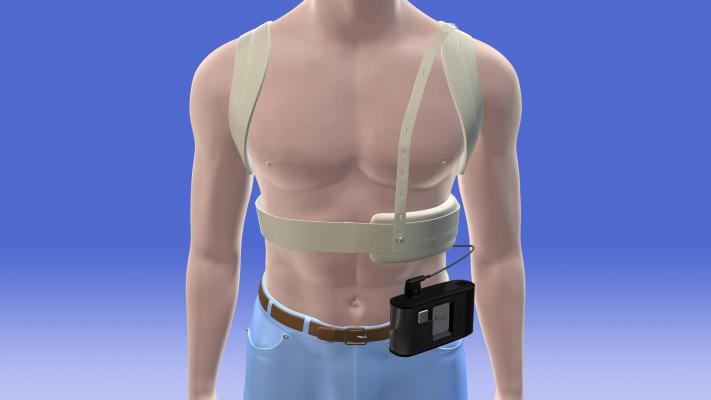
Wearable cardioverter defibrillator vest
- FDA approved wearable cardioverter defibrillator (WCD) vest, for patients who are at risk of Sudden Cardiac Death (SCD).
- Customer was looking for a overmoldable, fully encapsulated, medium powered vibration motor.
- In partnership, we designed an encapsulated motor, completely sealed, and validated it for low pressure overmolding.
Challenge
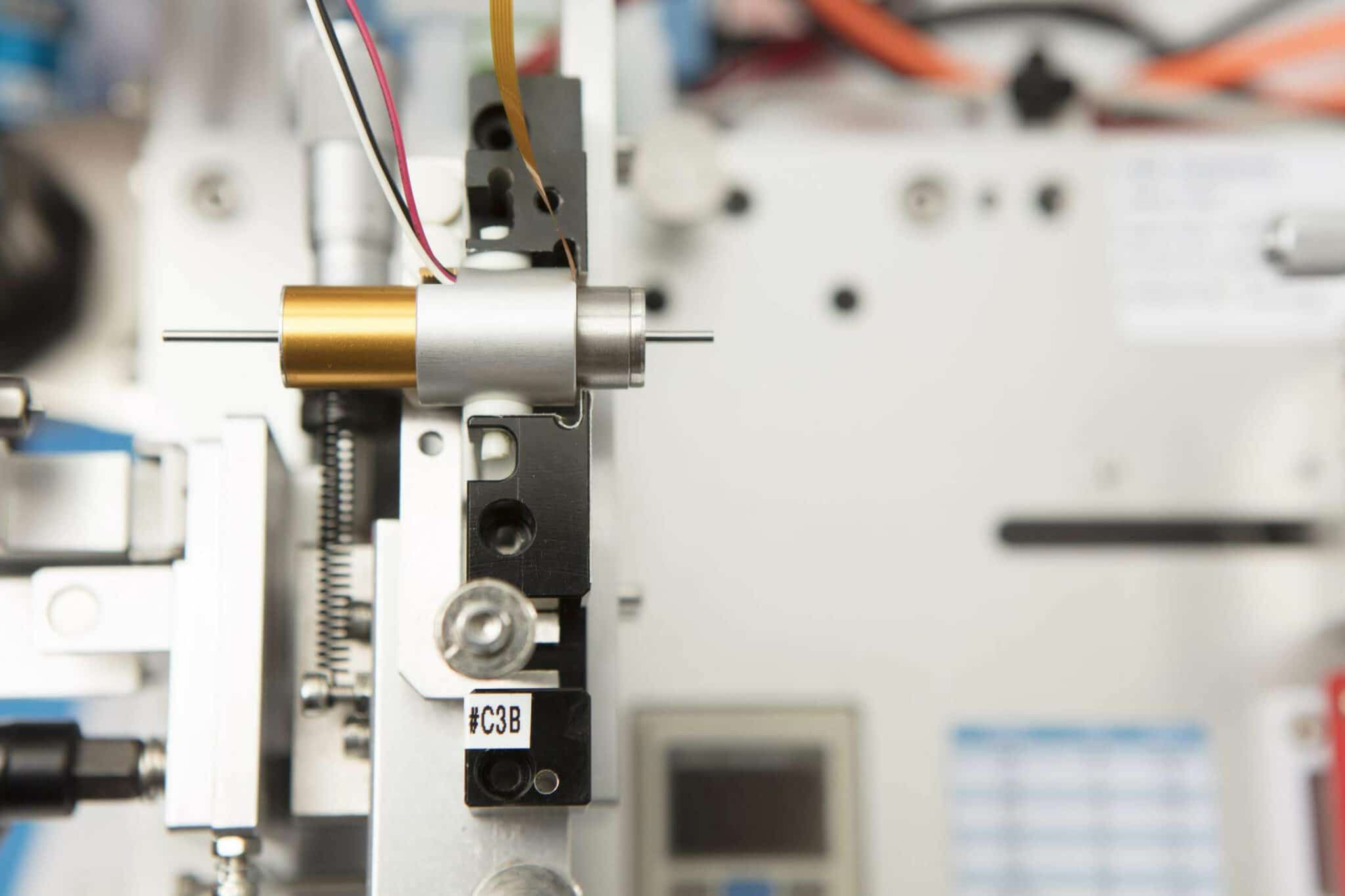
- Our client required a fully encapsulated, high reliability, medium powered vibration motor, for tactile vibration alerting.
- The vest had to be washable and therefore it was important to prevent ingress of water into the motor. The eccentric rotating mass was free to rotate by virtue of the hard-case encapsulation. When worn the vibration had to be strong enough to penetrate any fabric and clothing worn.
- Pricing was flexible but reliability was an area of concern for our client.
Solution
- We worked closely with the customers team to develop two possible motor solutions.
- After testing both solutions, we manufactured an encapsulated vibration motor, which was then further sealed, for complete IP68 protection against water ingression.
- We worked with the customer to validate the end solution fully, before preparing manufacturing jigs and processes to go into mass production.
Results
- A custom vibration motor was developed that addressed the unique design problems, faced by the customer.
- The device received FDA Certification and has has been successfully launched into the trial market with secondary market with their launch dates brought forward.
- We have an ongoing relationship with the customer to support their future projects.
Project scope
Tactile vibration alerting with a high realiability
Our customer approached us as they were designing a FDA approved wearable cardioverter defibrillator (WCD) vest, for patients who are at risk of Sudden Cardiac Death (SCD).
These patients could not have a surgically cardioverter defibrillator (ICD) fitted, and therefore the defibrillator has to be worn externally. The device monitors the heartrate patterns of the wearer and, and in the case that defibrillation event is needed, the unit sends an audible and tactile warning to the user. This is so that user can pull over, if for example, they’re driving.
As such, it’s very important that the vibration motor is reliable, particularly after the vest has been washed, and that’s where the need for full IP68 encapsulation was needed. The motor was to be further overmoulded as part of the application, so it also had to withstand the heat generated by the overmoulding process.
We worked with the customer to develop a bespoke solution, and after extensive validation we brought the module into ongoing mass production.
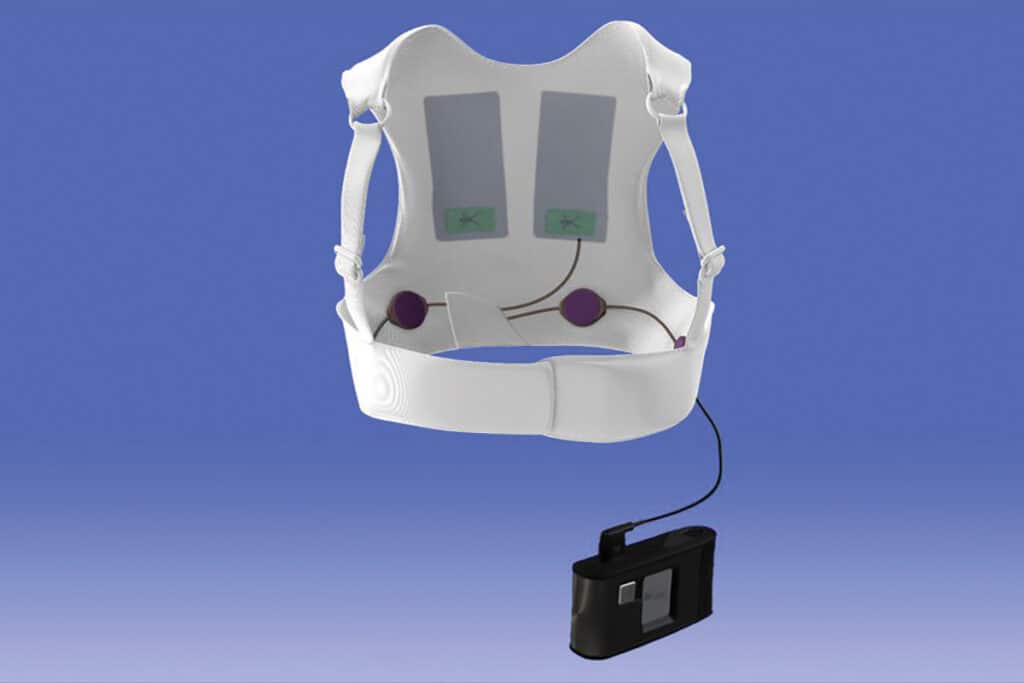

STAGE 1
Working together
- Our client required a fully encapsulated medium powered and highly reliable vibration motor.
- It was important to reduce the risk of the motor coming into contact with any moisture and material as part of the overmould process.
- The customer was flexible on price but had high quality expectations. Their primary concern was the solution meeting the reliability needs of the application.
- Working with their design team, our engineers built a specification based on shared input and ideas to create a collaborative partnership.
STAGE 2
Motor design and testing
- Their team were open to the changes in design we suggested to overcome production challenges.
- After testing, our solution was an encapsulated motor, fully sealed, creating an IP68 unit which could then be overmolded by the customer, in their application.
- We developed and manufactured a completely sealed vibration motor with circuitry encapsulated, totally removing the risk that material would ingress, and offering a further line of defence against water, when the vest was washed.
STAGE 3
Results and relationship
- The device received FDA certification, and the product has been successfully launched into the trial market. The launch date for a secondary market has been brought forward.
- Our customer was pleased with our collaborative approach, and we were able to develop a solution that met their exact needs and specification.
- We also felt that there would be a need for this type of fully encapsulated motor in other market sectors, so we have added an adapted version to our catalogue as a standard part, enabling us to offer it as a solution to other customers.
CASE STUDIES
We supply motor and mechanism solutions for all sorts of applications
-
Medical patient simulator
Patient simulator for first-aid cardiac arrest training required a vibration motor to haptic simulate a heartbeat.
View Case Study
-
Swarm robot research linear actuator

Challenging technical and unique application for swarm robotics for a European robotic research Institution.
View Case Study
-
Bench-top pill counting machine

Bench-top medical instrument used a vibration motor to aid movement of pills through instrument pathways.
View Case Study
-
Weather balloon measurement lab

High volumes of low-cost reliable single-use motors for weather balloon measurement lab.
View Case Study
-
Emergency services ruggedised radio

Emergency radios to withstand high temperatures, impacts, and vibrate through protective clothing
View Case Study
-
Surgically implanted pacemaker motor
Brushless vibration motor within a FDA Class III implanted medical pacemaker device.
View Case Study
MORE THAN YOUR AVERAGE MOTOR SUPPLIER
We manage your risk
We manage your risk through optimised design and rigorous testing, protecting your application and intellectual property, fulfilling your performance requirements whilst reducing your lifecycle costs.
MOTORS & MECHANISMS
Precision products
From off the shelf motor components to fully validated and tested complex mechanisms, choose a motor and mechanism partner you can trust.
-
Mechanisms
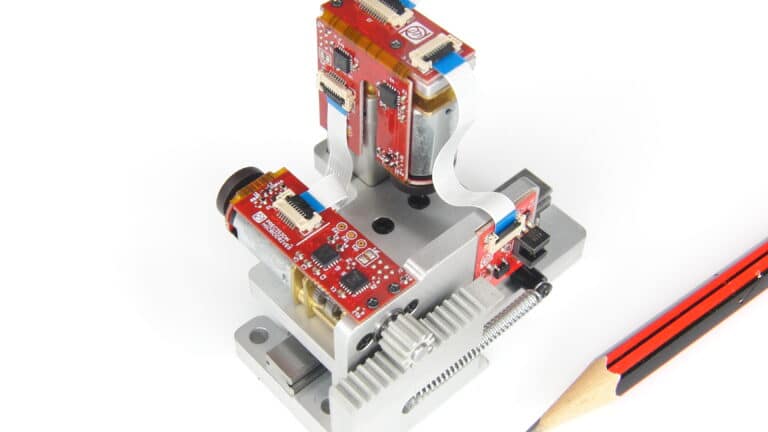
Custom motor assemblies designed and manufactured to your requirements. A turnkey service from design inputs to mass production.
View Page
-
Vibration motors

With every vibration and haptic technology covered, we’ll help you select the best vibrating solution for your application.
View Page
-
DC motors

Iron-core, coreless and brushless DC motor technologies in a wide range of form factors and sizes, with off the shelf
View Page
-
DC gear motors
Building on our range of DC motors, we integrate spur and planetary gearboxes, from 6mm to 60mm frame diameters.
View Page
-
Haptic feedback

Practical and reliable, low noise and high quality haptic feedback solutions for all types of application user interface.
View Page
Discover more
Resources and guides
Discover our product application notes, design guides, news and case studies.
Industries we serve
At Precision Microdrives we design and manufacture customised electric motors and mechanisms across a number of industries.
Precision Microdrives
Whether you need a motor component, or a fully validated and tested complex mechanism – we’re here to help.